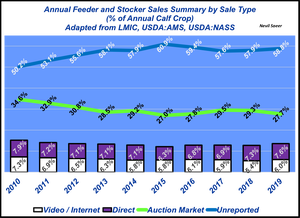
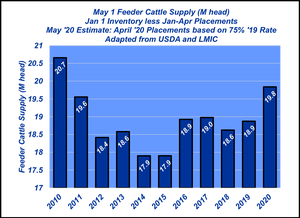
Stocker backgrounding
thumbnail
Farm Business Management
SDSU Extension hosting webinar on backgrounding calvesSDSU Extension hosting webinar on backgrounding calves
Series will provide the most up-to-date research.
Subscribe to Our Newsletters
BEEF Magazine is the source for beef production, management and market news.